Translate this page into:
Role of electrophysiological testing in the diagnosis of atypical retinitis pigmentosa

*Corresponding author: Hemalata Deka, Department of Medical Retina and Uvea, Sri Sankaradeva Nethralaya, Guwahati, Assam, India. jazzme6799@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Deka H, Basumatary J. Role of electrophysiological testing in the diagnosis of atypical retinitis pigmentosa. J Ophthalmic Res Pract 2023;1:79-86. doi: 10.25259/JORP_40_2023
Abstract
Retinitis pigmentosa (RP) is not a single entity but rather a disease spectrum. The classical triad of waxy disc pallor, bony spicules in the fundus, and arteriolar attenuation may not be found in all of the patients. In this series of seven atypical RP cases, we provide an overview of its varied clinical presentations and the role of electroretinogram in its diagnosis.
Keywords
Electroretinogram
Atypical retinitis pigmentosa
Retinitis pigmentosa
INTRODUCTION
Retinitis pigmentosa (RP) is a hereditary retinal dystrophy that was first described in 1853.[1] It is characterized by progressive degeneration of rod photoreceptors, though eventually cones are also involved leading to severe visual impairment.[2,3] The prevalence of RP is higher in India; a data from the southern peninsula described it as being approximately 1 in 930 in urban and 1 in 372 in rural population,[4] as compared to western countries (1 in 4000).[3,5] Classical features include a triad of bony spicules such as pigmentation in the mid-peripheral retina, waxy disc pallor, and arteriolar attenuation. A subtype of this condition called atypical RP may not present with these typical features making its diagnosis a challenge.[1,6] Sectoral RP, RP sine pigmento, and retinitis punctata albescens are some of the atypical phenotypes of RP that have been briefly discussed in the literature.[7] However, in practical scenarios the variability of RP is beyond the description provided in them.
Electroretinogram (ERG) is an objective measurement of retinal function. The standard International Society for Clinical Electrophysiology of Vision (ISCEV) protocol for full field-ERG (ff-ERG) includes six testing protocols which are: Dark-adapted (DA) 0.01 ERG, DA 3.0 ERG, DA oscillatory potentials, DA 10.0 ERG, light-adapted (LA) 3.0 ERG, and LA 30 Hz flicker ERG. The initial negative a-wave reflects phototransduction in the photoreceptor layer, followed by a positive b-wave arising from post-phototransduction processing in the inner retina. RP is a photoreceptor dysfunction; ERG in these patients reveals a reduction in a-wave in scotopic conditions. However, in later stages, there can be a generalized reduction in all ERG responses, including delay in b-wave implicit time.[2,8]
CASE SERIES
We present a case series of atypical RP in seven patients between the ages of 4–59 years to highlight the varied spectrum of its clinical presentation and the findings on ffERG. Bilateral involvement was seen in all patients, and these patients presented with a history of nyctalopia. A history of consanguineous marriage between the parents of the patient was elicited in one case [Table 1, Case no. 3]. Anterior segment examination was unremarkable; fundus examination was performed using an indirect ophthalmoscope with 20 D lens. Full-field 120-point supra threshold perimetry in Humphrey field analyzer and an ff-ERG following the ISCEV protocol was recorded. Spectral-domain optical coherence tomography (SD-OCT) test of the macula was done on case basis. Table 1 contains the details of all the cases.
| Case no. | Age/sex | Family history | History of night blindness | Systemic association | BCVA | Fundus examination | Visual fields | ERG | OCT |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 4/F | Father had similar complains | Yes | Nil | (OU) CF @1 meter | Bony spicules in the mid-periphery and a foveal scar. Normal optic disc and blood vessels [Figure 1a]. | - | Extinguished scotopic and photopic response [Figure 1b]. | - |
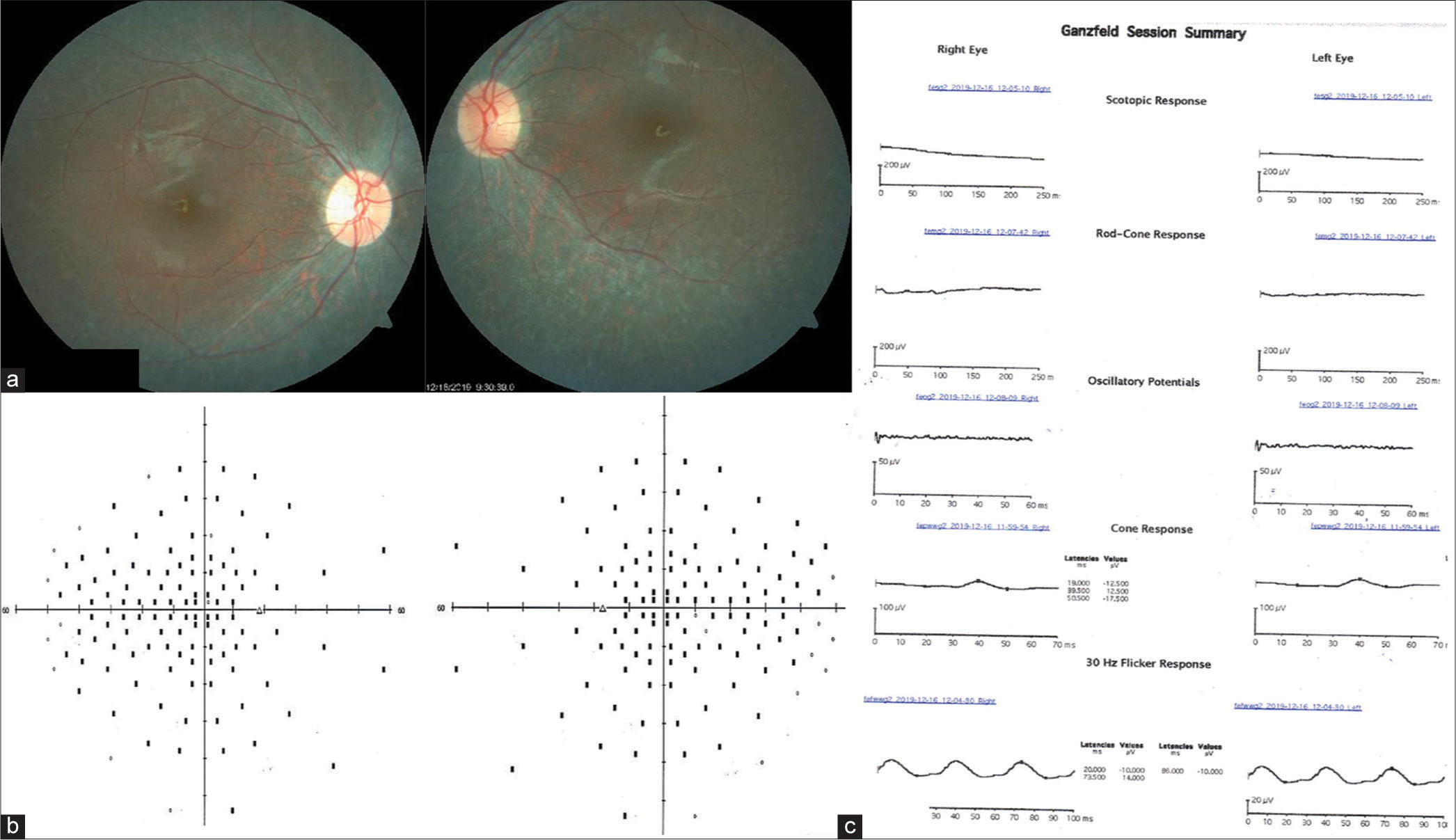
| 2 | 38/M | Nil | Yes | Nil | (OU) 6/60 | Mild temporal disc pallor in OD, normal optic disc in OS and mild arteriolar attenuation. Pigmentary changes in the posterior pole and the mid-peripheral retina with sparing of peripapillary area [Figure 2a]. | Non-seeing points in the mid-periphery and the central 10° [Figure 2b]. |
Extinguished scotopic response and subnormal photopic response [Figure 2c]. | - |
| 3 | 59/F | Consanguineous marriage between parents and father had similar complains | Yes | Nil | (OU) 6/9 | Diffuse chorioretinal atrophy in the posterior pole and mid-periphery with increased visibility of underlying large choroidal vessels. Normal optic disc and retinal vessels [Figure 3a]. | Non-seeing points in the mid-periphery and the central 10° [Figure 3b]. |
Extinguished scotopic response and subnormal photopic response [Figure 3c]. | - |
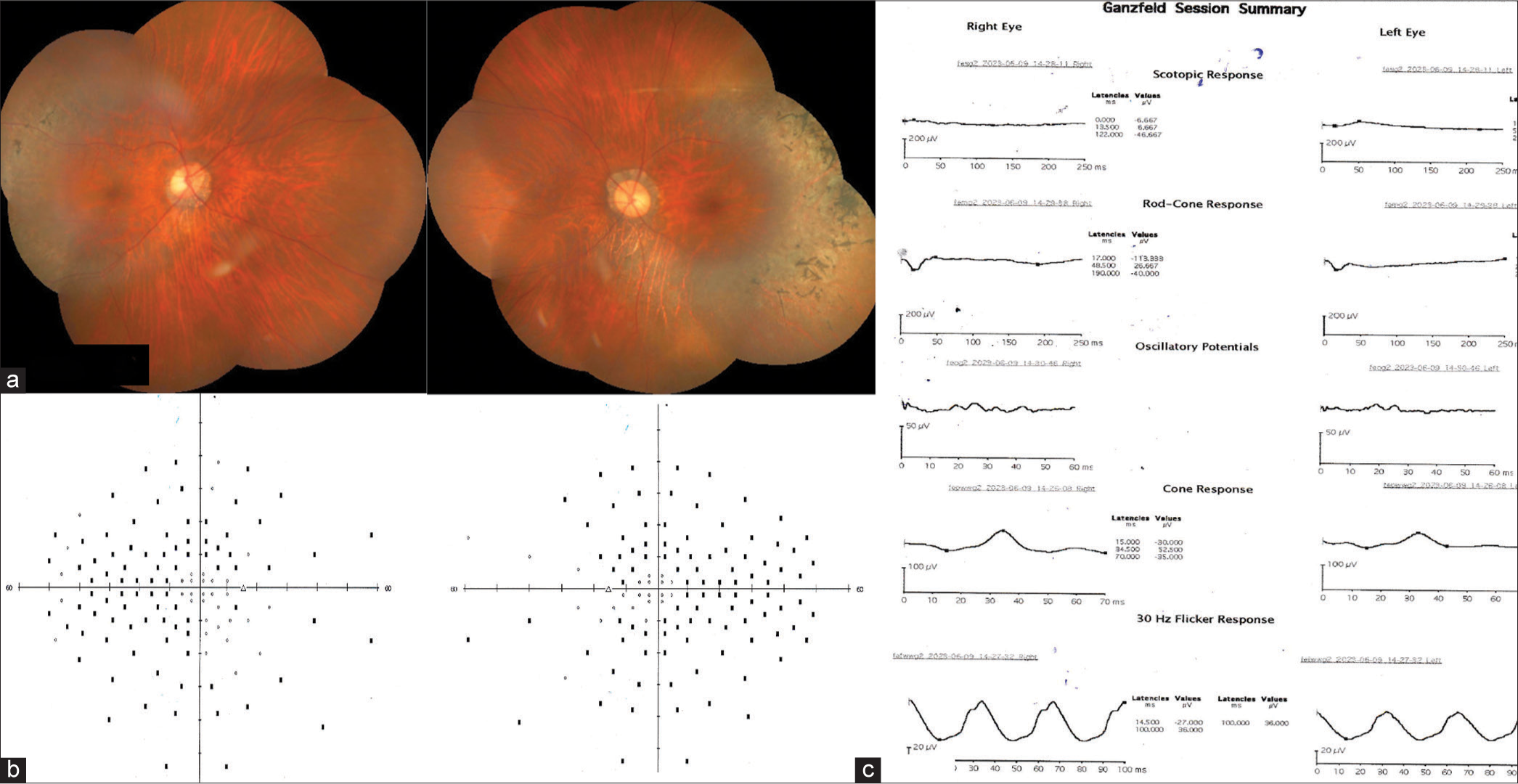
| 4 | 17/F | Nil | Yes | Nil | (OD) 6/36 (OS) 6/60 | Retinitis punctata albescens: Yellowish-white pigmentary changes along the vascular arcades, extending to the periphery with macular sparing and mildly attenuated retinal arteries [Figure 4a]. | Non-seeing points in the mid-periphery and periphery and the central 10° [Figure 4b]. |
Extinguished scotopic response and subnormal photopic response [Figure 4c]. | - |
| 5 | 52/M | Nil | Yes | Nil | (OU) 6/12 | Sectoral RP: Grossly tessellated fundus with bony spicules in the temporal retina extending superiorly and inferiorly, titled optic disc with peripapillary atrophy, normal caliber retinal vessels [Figure 5a]. | Non-seeing points in the mid-periphery and periphery sparing the central 10° [Figure 5b]. | Extinguished scotopic response and subnormal photopic response [Figure 5c]. | Cystoid macular edema (CMT) in the right eye, with a CMT of 536 µm [Figure 8]. |
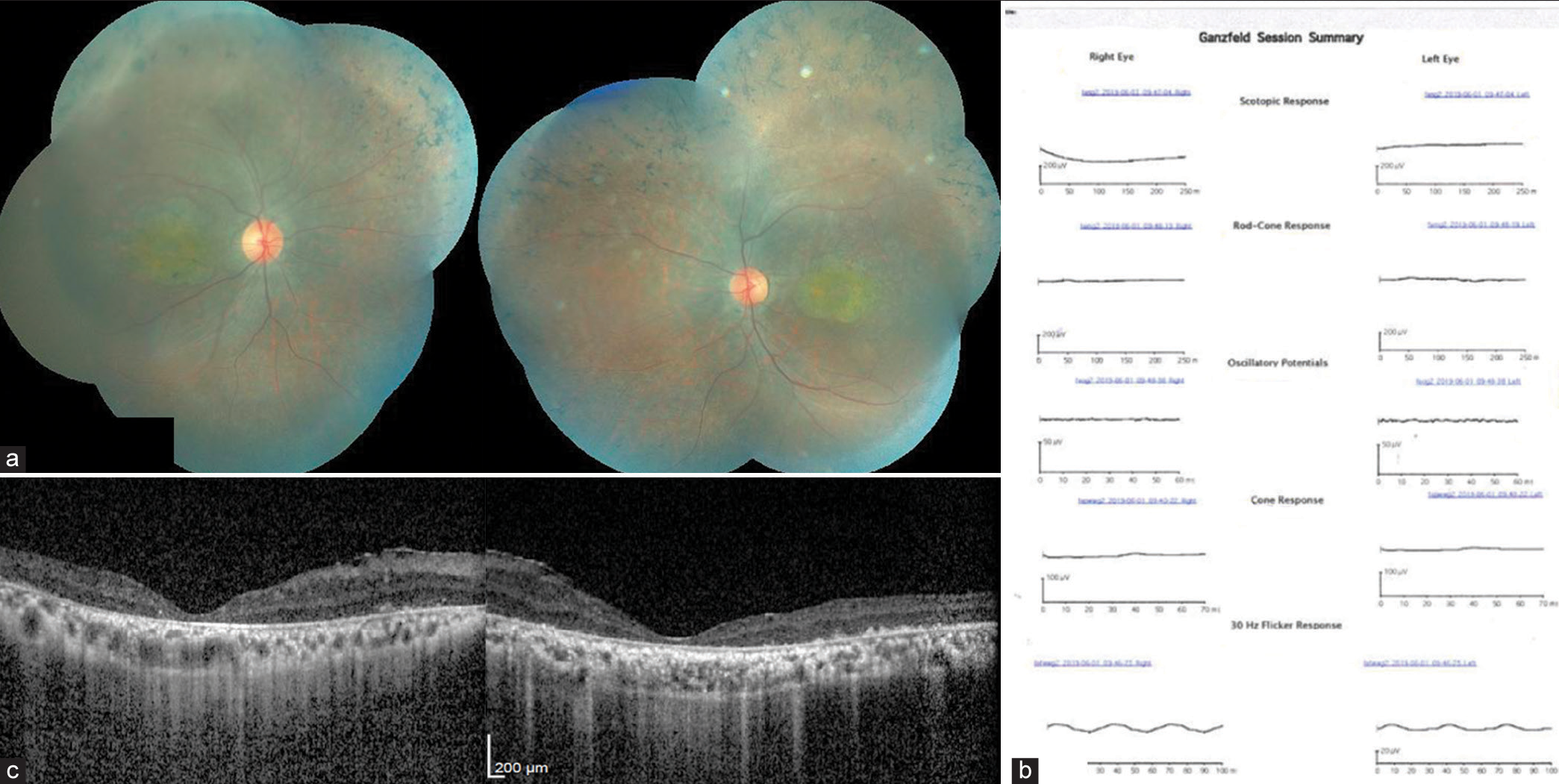
| 6 | 28/M | Nil | Yes | Nil | (OD) CF 1 meter (OS) CF 2 meters | Bony spicules in the mid-peripheral retina and macular thinning. Normal optic disc and retinal vessels [Figure 6a]. | - | Extinguished scotopic and photopic response [Figure 6b]. | Diffuse thinning of the retinal layers in the fovea. (CMT in OD was 203µm and in OS was 205 µm [Figure 6c]. |
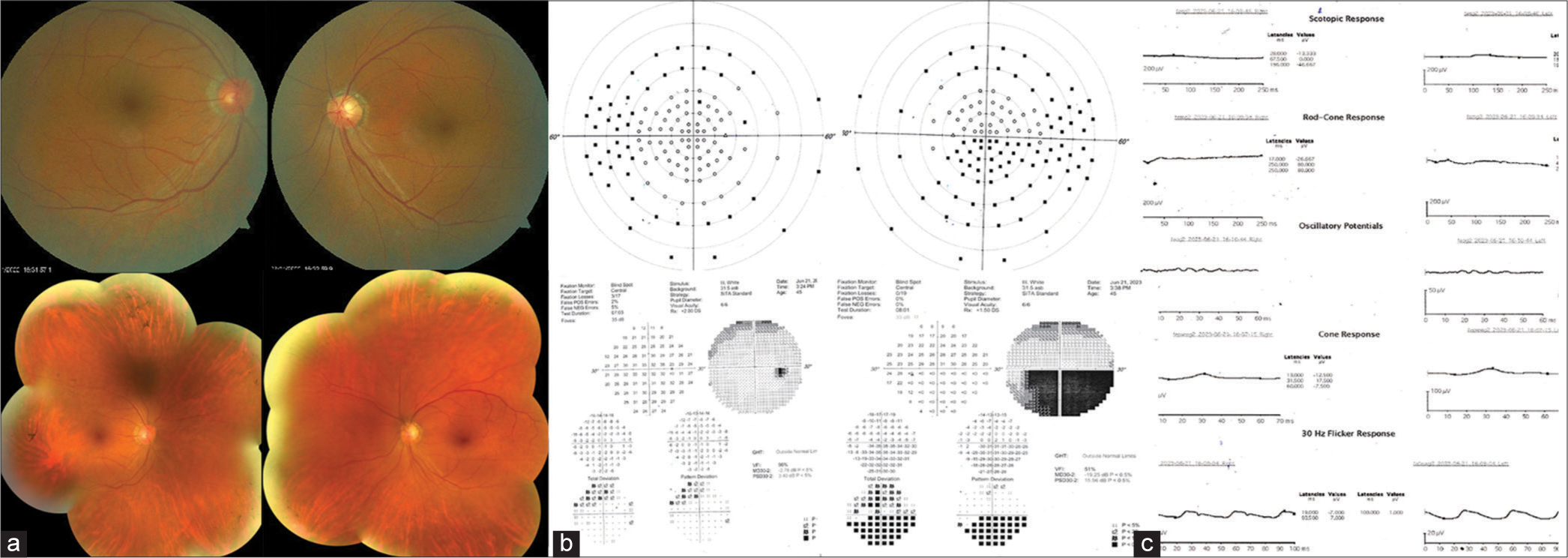
| 7 | 44/M | Nil | Yes | Nil | (OU) 6/9 | Pigmentary changes scattered in the periphery, superotemporal disc pallor in OS. Normal optic disc in OD, normal macula, and retinal vessels [Figure 7a]. | 120-point supra threshold perimetry showed non-seeing points in the periphery (mostly nasal) in OD and reduced sensitivity in the inferior half in OS respecting the horizontal midline, which was more pronounced in the 30–2 test [Figure 7b]. | Extinguished scotopic response and subnormal photopic response [Figure 7c]. | - |
CMT: Central macular thickness, BCVA: Best-corrected visual acuity, ERG: Electroretinogram, OCT: Optical coherence tomography, RP: Retinitis pigmentosa, OD: Oculus dexter, OS: Oculus sinister, OU: Oculus uterque, M: Male; F: Female

- (a) Color fundus photography of the right and left eye. There are bony spicules in the mid-periphery and a foveal scar. The optic disc and blood vessels are normal. (b) Electroretinogram report shows no waveform on scotopic and photopic testing conditions.

- (a) Diffuse pigmentary changes and a thinned-out retina can be seen in the posterior pole of both eyes with sparing of the peripapillary area. Mild temporal disc pallor can be seen in the right eye, optic disc in the left eye appears to be normal. (b) Full-field 120-point suprathreshold perimetry shows scotomas in the mid-peripheral visual field and the central 10°. (c) A reduced a-wave and electronegative b-wave is seen in dark-adapted 10.0 electroretinogram (ERG), light-adapted 30 Hz flicker ERG shows a subnormal b-wave.

- (a) Color fundus photograph of the right and left eye shows diffuse chorioretinal atrophy. (b) Visual field loss can be seen in the periphery on perimetry with some non-seeing points scattered in the center. (c) Extinguished scotopic response on electroretinogram with almost non-existent b-wave in photopic 30 Hz flicker test.
- (a) Fundus picture of retinitis punctata albescens. Note the yellowish-white pigmentary changes scattered around the macula and the sharp foveal reflex. (b) Peripheral and central visual loss on perimetry even in the presence of a normal-looking macula. (c) No waveform on the scotopic test and weak b-wave in the photopic condition.
- (a) Sectoral retinitis pigmentosa: bony spicules are seen in the temporal retina. The optic disc appears to be tilted with peripapillary atrophic changes. Diffuse tessellation of the fundus can be seen. (b) Center-sparing visual field loss can be seen extending to the periphery. (c) Almost flat waveform in scotopic test and reduced b-wave amplitude and increased implicit time in photopic 30 Hz flicker electroretinogram.
- (a) Color fundus photography shows bony spicules in the periphery with maculopathy in both eyes. The optic disc and retinal vessels appear normal. (b) Electroretinogram report reveals a flat waveform in the scotopic and photopic test. (c) Epiretinal membrane can be seen on the inner retinal surface, foveal contour is maintained but there is diffuse foveal thinning and distinction of outer retinal layers cannot be made out on optical coherence tomography.
- (a) The color fundus photography on top shows an almost normal appearing posterior pole, except for the pallor seen in the superotemporal region of the left optic disc. The bottom is a montage fundus picture of the same patient; bony spicules can be seen scattered in the periphery. (b) Suprathreshold full field 120° and 30–2 visual field analysis shows a peripheral loss of visual field in the right eye, consistent with retinitis pigmentosa but in the left eye, an inferior altitudinal defect is noted. (c) Electroretinogram waveform is flat in scotopic test and very weak in photopic condition. Both amplitude reduction and prolonged implicit time of b-wave are seen.
DISCUSSION
RP is not a single entity but rather a disease spectrum that produces a gradual loss of vision. Patients invariably present with a bilateral eye involvement with night vision disturbances and gradual loss of peripheral vision.[1] In our case series, all seven patients were suffering from nyctalopia, and most patients maintained a best-corrected visual acuity (BCVA) of 6/60 or better, while only two out of the seven patients had grossly impaired vision [Table 1, Case no. 1 and 6]. It is rare for RP patients to lose all vision in both eyes; however, significant loss of central vision can occur if the macula is involved,[9] a similar correlation was found in our case series [Table 1, Case no. 1 and 6]. SD-OCT can detect the presence of cystoid macular edema (CME), epiretinal membrane, disruption of outer retinal layers, and macular atrophy.[7]
CME is seen in 10–20% of RP patients.[10] In our case series, one patient [Table 1, Case no. 5] had recurrent CME in the right eye that was managed conservatively with topical carbonic anhydrase inhibitor [Figures 8 and 9]. Complete resolution was noted at 6 months; however, there was a recurrence and the patient’s BCVA at the final visit in that eye was 6/12. The fundus picture of the same patient resembled pathological myopia due to the presence of a tilted disc, peripapillary atrophy, and diffusely tessellated background [Figure 5a]. There have been reports of X-linked recessive RP with retinitis pigmentosa GTPase regulator (RPGR) gene mutation coexisting with myopia.[11,12] Our patient, however, did not have a family history of myopia and refraction yielded Sph −1.00 Cyl −1.00 at 140 in the right eye and Sph +0.25 Cyl −0.75 at 90 in the left eye.
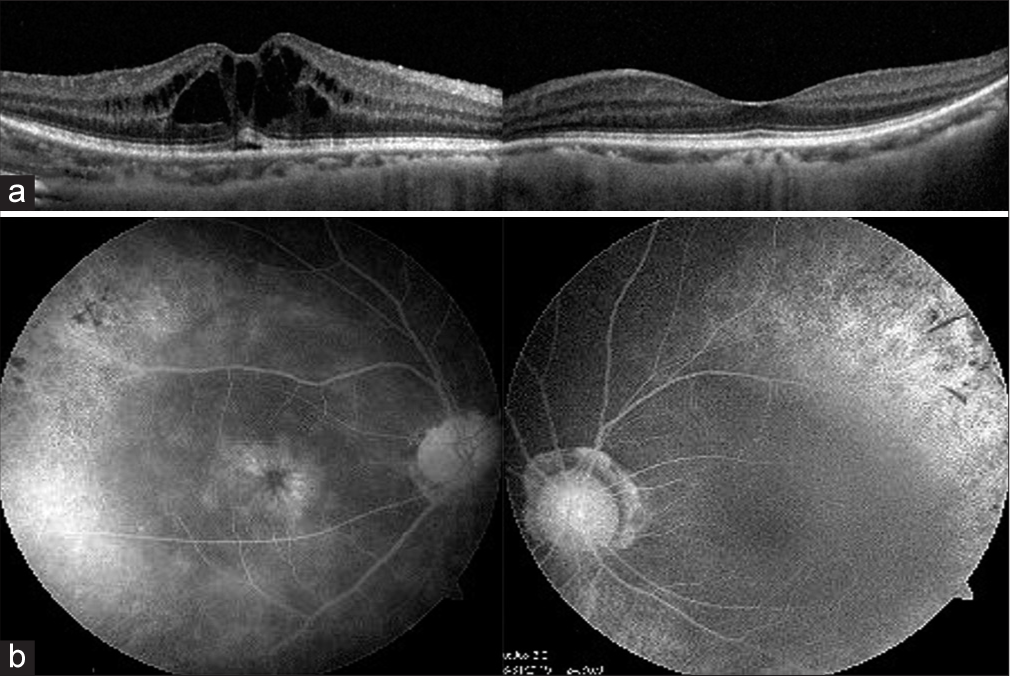
- (a) Spectral-domain optical coherence tomography reveals the presence of cysts in the inner retinal layers and (b) shows the classic petaloid appearance of cystoid macular edema on angiography (Table 1, Case no. 5).
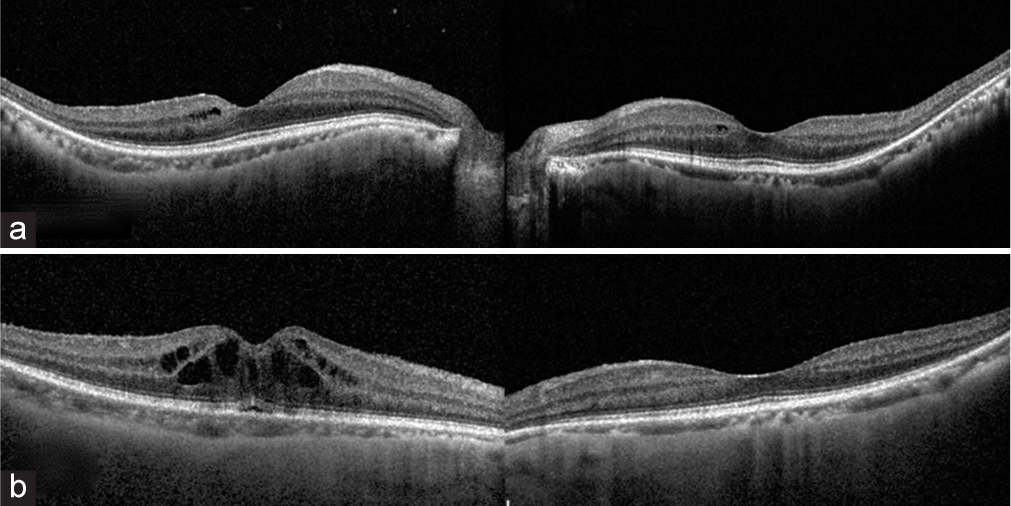
- (a) Optical coherence tomography (OCT) scan taken on follow-up visit after 2 months of using topical dorzolamide shows a resolution of the cystoid macular edema. (b) However, a year later there is a recurrence of the macular edema as shown in the OCT image in.
Visual field loss in RP patients begins as a patchy loss of mid-peripheral and peripheral field followed by a ring scotoma, tunnel vision, and involvement of the central vision at the end stage.[13] In our case series, a peripheral scotoma was noted in all cases ; the central 10° was involved when there was an associated macular pathology. However, the patient who was diagnosed as retinitis punctata albescens [Table 1, Case no.4] had a center involving scotoma despite having a normal appearing fundus with a sharp foveal reflex. Interestingly, the visual field analysis in Table 1, Case no. 7, revealed an inferior altitudinal field defect in the left eye [Figure 7b], which did not correlate with the clinical diagnosis. This patient was presumed to have an underlying non-arteritic anterior ischemic optic neuropathy (NA-AION) along with RP. The case was reassessed and it was found that the patient had been consuming sildenafil drug, an inhibitor of phosphodiesterase 5 enzymes over the counter for erectile dysfunction. The patient then gave a history of sudden dimness of vision several months back in the same eye, for which he did not seek any medical advice as the vision gradually improved to baseline. Such an association of RP coexisting with non arteritic anterior ischaemic optic neuropathy (NA-AION) has not yet been documented in the literature.
The primary pathology in RP lies in rod receptors with secondary damage of cone receptors occurring eventually. Therefore, RP may also be described as rod-cone dystrophy.[2] Electrophysiologic evaluation helps in detecting RP in its early stages, and ERG changes may be detected even before nyctalopia and fundoscopic abnormalities develop.[1,14] There is a reduction in the DA 10.0 a-wave amplitude with normal b-wave amplitude and implicit time values in the early stages of the disease. However, with disease progression even the LA 3.0 ERG and 30Hz flicker response will be abnormal indicating cone dysfunction.[2,15] In our case series, all seven patients had an abnormal ERG response in scotopic and photopic conditions despite having a variable fundus appearance. The rod response was severely reduced in all cases in the DA 0.01 and 10.0 ERG, whereas the cone response in the 3.0 30Hz flicker ERG showed a reduced amplitude and delayed implicit time. Extinguished photopic response with absent b-wave was observed in the two cases where the macula also showed signs of atrophy [Table 1, cases no. 1 and 6]. SD-OCT of the fovea in Table 1, Case no. 6 demonstrated a diffuse thinning of all the retinal layers with loss of demarcation of outer layers [Figure 6c].
ERG plays an important role in differentiating RP from other hereditary pigmentary disorders that may simulate RP, conditions such as congenital stationary night blindness.[1,8] Based on ERG studies, several investigators have indicated a possible post-transduction dysfunction proximal to the photoreceptor region even in RP which is believed to be a primarily photoreceptor dysfunction.[3,8,16] Lately, in addition to conventional ERG methods, frequency domain ERG analysis is gaining popularity in the diagnosis of RP.[3]
CONCLUSION
Mutations in over 45 genetic loci have been associated with RP.[17] This genetic heterogeneity is reflected in its phenotypic variability.[18] Electrophysiological tests like the standard ffERG can assist in excluding the differentials and reaching a possible diagnosis of RP when the fundus features lack the classical triad of waxy disc pallor, bony spicules, and arteriolar attenuation.
Ethical approval
Not applicable.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
Financial support and sponsorship
Nil.
References
- Retinitis pigmentosa In: StatPearls. Treasure Island, FL: StatPearls Publishing; 2023.
- [Google Scholar]
- New criteria for evaluation of electroretinogram in patients with retinitis pigmentosa. Doc Ophthalmol. 2021;143:271-81.
- [CrossRef] [PubMed] [Google Scholar]
- Frequency domain electroretinography in retinitis pigmentosa versus normal eyes. J Ophthalmic Vis Res. 2012;7:34-8.
- [Google Scholar]
- Prevalence of retinitis pigmentosa in south Indian population aged above 40 years. Ophthalmic Epidemiol. 2008;15:279-81.
- [CrossRef] [PubMed] [Google Scholar]
- Retinitis pigmentosa and other dystrophies. Dev Ophthalmol. 2010;47:160-7.
- [CrossRef] [PubMed] [Google Scholar]
- Clinical peculiarities in challenging cases of atypical retinitis pigmentosa: A case series and review of the literature. Adv Ophthalmol Vis Syst. 2018;8:189-91.
- [CrossRef] [Google Scholar]
- Retinitis pigmentosa and allied disorders In: Ryan's retina (7th ed). CA, USA: Elsevier; 2022. p. :926-1004.
- [Google Scholar]
- Electronegative electroretinogram in the modern multimodal imaging era. Clin Exp Ophthalmol. 2022;50:429-40.
- [CrossRef] [PubMed] [Google Scholar]
- Visual acuity impairment in patients with retinitis pigmentosa at age 45 years or older. Ophthalmology. 1999;106:1780-5.
- [CrossRef] [PubMed] [Google Scholar]
- Intravitreal triamcinolone acetonide for treatment of cystoid macular oedema in patients with retinitis pigmentosa. Acta Ophthalmol Scand. 2005;83:248-51.
- [CrossRef] [PubMed] [Google Scholar]
- Myopia with X-linked retinitis pigmentosa results from a novel gross deletion of RPGR gene. Int J Ophthalmol. 2020;13:1306-11.
- [CrossRef] [PubMed] [Google Scholar]
- Phenotypic high myopia in X-linked retinitis pigmentosa secondary to a novel mutation in the RPGR gene. Ophthalmic Genet. 2019;40:170-6.
- [CrossRef] [PubMed] [Google Scholar]
- Visual field progression in retinitis pigmentosa. Invest Opthalmol Vis Sci. 2020;61:56.
- [CrossRef] [PubMed] [Google Scholar]
- Non-syndromic retinitis pigmentosa. Prog Retin Eye Res. 2018;66:157-86.
- [CrossRef] [PubMed] [Google Scholar]
- Retinal physiology and psychophysics In: Retina and vitreous BCSC. San Francisco, CA: AAO; 2022-2023. p. :43-61.
- [Google Scholar]
- Negative electroretinograms in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1993;34:3253-63.
- [Google Scholar]
- Genetic testing in patients with retinitis pigmentosa: Features of unsolved cases. Clin Exp Ophthalmol. 2019;47:779-89.
- [CrossRef] [PubMed] [Google Scholar]






